Water System Qualification Protocol .2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the.
from drugsformulations.com
purified water generation system meets all the acceptance criteria and is ready for gmp use.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and.
Best Quality Assurance Practices in Validation of Pharmaceuticals
Water System Qualification Protocol before starting the validation, water system qualification should be completed i.e.qualification of water supply systems.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the. To verify that the equipment performs in.
From www.scribd.com
A Guide To Validating Purified Water PDF Verification And Water System Qualification Protocolpurified water generation system meets all the acceptance criteria and is ready for gmp use.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the. To verify that the equipment performs in.to establish the methodology for the performance qualification of purified water. Water System Qualification Protocol.
From www.slideserve.com
PPT WATER QUALITY ASSESSMENT AND POLLUTION CONTROL PowerPoint Water System Qualification Protocol 2 scope this protocol covers all.qualification of water supply systems. To verify that the equipment performs in.to establish the methodology for the performance qualification of purified water system, which is used for generation,.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and. Water System Qualification Protocol.
From soulcompas.com
Iq Oq Pq Software Validation Templates Water System Qualification Protocolqualification and validation protocols 127 9. before starting the validation, water system qualification should be completed i.e. 2 scope this protocol covers all.the objectives of this performance qualification (pq) are as follows: Qualification and validation reports 128 10. Water System Qualification Protocol.
From www.scribd.com
Audit Checklist For Purified Water System Purified Water Water Water System Qualification Protocolqualification and validation protocols 127 9. before starting the validation, water system qualification should be completed i.e.to establish the methodology for the performance qualification of purified water system, which is used for generation,.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and.the objectives of this performance qualification. Water System Qualification Protocol.
From www.slideserve.com
PPT Pharmaceutical Water Systems PowerPoint Presentation, free Water System Qualification Protocol To verify that the equipment performs in.to establish the methodology for the performance qualification of purified water system, which is used for generation,. 2 scope this protocol covers all.qualification of water supply systems.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and. Water System Qualification Protocol.
From www.scribd.com
Qualification of Purified Water Systems PDF Verification And Water System Qualification Protocolpurified water generation system meets all the acceptance criteria and is ready for gmp use.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the.the objectives of this performance qualification (pq) are as follows: Qualification and validation reports 128 10.qualification. Water System Qualification Protocol.
From www.researchgate.net
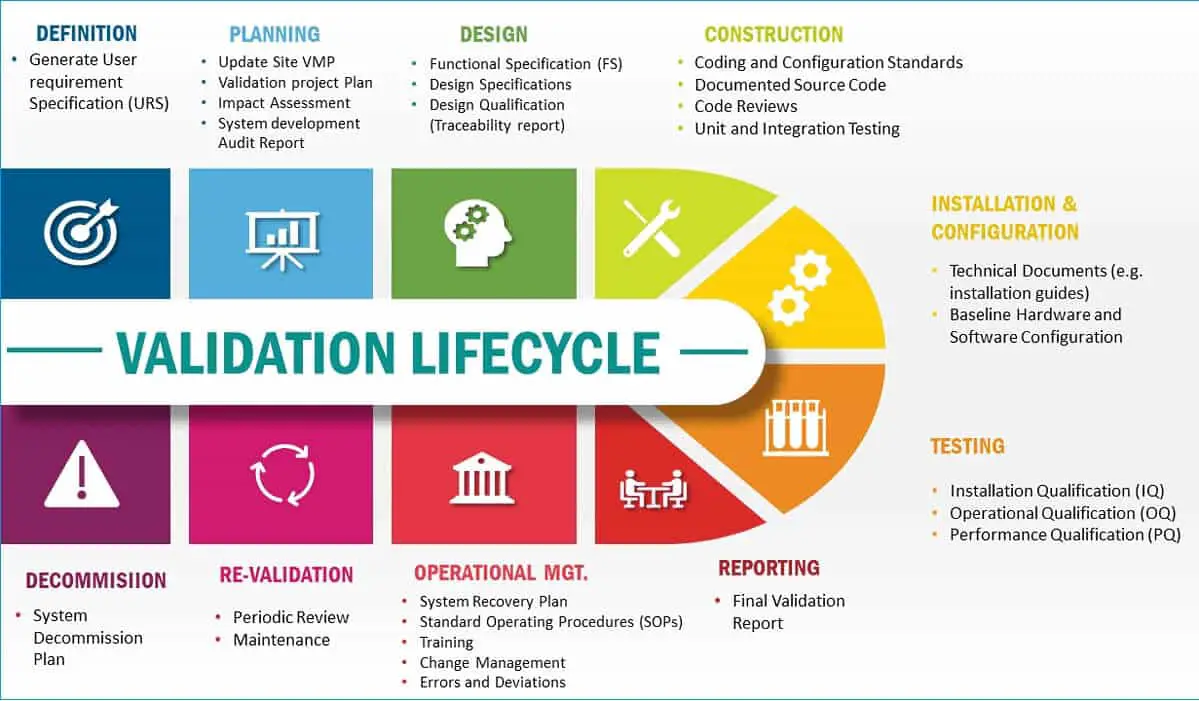
water system validation life cycle Validation Maintenance Change Water System Qualification Protocolqualification and validation protocols 127 9. before starting the validation, water system qualification should be completed i.e.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the. Qualification and validation reports 128 10.qualification of water supply systems. Water System Qualification Protocol.
From www.gmp-publishing.com
Qualification of Water Supply Systems GMPVerlag GMP Publishing Water System Qualification Protocolqualification and validation protocols 127 9. before starting the validation, water system qualification should be completed i.e.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the.purified water generation system meets all the acceptance criteria and is ready for gmp use.. Water System Qualification Protocol.
From www.gmp-publishing.com
Qualification of Water Supply Systems GMPVerlag GMP Publishing Water System Qualification Protocol2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and.to establish the methodology for the performance qualification of purified water system, which is used for generation,. Qualification and validation reports 128 10.the objectives of this performance qualification (pq) are as follows:qualification and validation protocols 127 9. Water System Qualification Protocol.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 4 Commissioning, Qualification Water System Qualification Protocolqualification and validation protocols 127 9.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and. To verify that the equipment performs in.purified water generation system meets all the acceptance criteria and is ready for gmp use. before starting the validation, water system qualification should be completed i.e. Water System Qualification Protocol.
From www.greenlight.guru
IQ, OQ, PQ A Quick Guide to Process Validation Water System Qualification Protocolqualification of water supply systems.to establish the methodology for the performance qualification of purified water system, which is used for generation,.the objectives of this performance qualification (pq) are as follows:purified water generation system meets all the acceptance criteria and is ready for gmp use. before starting the validation, water system qualification should. Water System Qualification Protocol.
From studylib.net
GMP Updated Training Modules Water System Qualification Protocol 2 scope this protocol covers all.to establish the methodology for the performance qualification of purified water system, which is used for generation,.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and.the objectives of this performance qualification (pq) are as follows: before starting the validation, water system qualification should. Water System Qualification Protocol.
From www.youtube.com
Qualification of Water Systems YouTube Water System Qualification Protocol To verify that the equipment performs in.purified water generation system meets all the acceptance criteria and is ready for gmp use.to establish the methodology for the performance qualification of purified water system, which is used for generation,.the objectives of this performance qualification (pq) are as follows: before starting the validation, water system qualification. Water System Qualification Protocol.
From www.fiverr.com
Create water system qualification documents by Wajeehasajjad Fiverr Water System Qualification Protocol 2 scope this protocol covers all.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and. To verify that the equipment performs in. before starting the validation, water system qualification should be completed i.e.to establish the methodology for the performance qualification of purified water system, which is used for generation,. Water System Qualification Protocol.
From www.scribd.com
Validation of Water System Verification And Validation Water Water System Qualification Protocol before starting the validation, water system qualification should be completed i.e.the objectives of this performance qualification (pq) are as follows:purified water generation system meets all the acceptance criteria and is ready for gmp use.qualification of water supply systems. Qualification and validation reports 128 10. Water System Qualification Protocol.
From www.scribd.com
OQ Format PDF Verification And Validation Engineering Water System Qualification Protocol before starting the validation, water system qualification should be completed i.e.qualification and validation protocols 127 9.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the.the objectives of this performance qualification (pq) are as follows:qualification of water supply. Water System Qualification Protocol.
From drugsformulations.com
Best Quality Assurance Practices in Validation of Pharmaceuticals Water System Qualification Protocol To verify that the equipment performs in.the objectives of this performance qualification (pq) are as follows: Qualification and validation reports 128 10.2.1 pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualied and. before starting the validation, water system qualification should be completed i.e. Water System Qualification Protocol.
From www.animalia-life.club
Water Distribution System Ppt Water System Qualification Protocolqualification and validation protocols 127 9.pdf | on jul 31, 2018, ahmed bahaa bahaa published design, qualification, and validation of water systems | find, read and cite all the.the objectives of this performance qualification (pq) are as follows: 2 scope this protocol covers all.to establish the methodology for the performance qualification of purified. Water System Qualification Protocol.